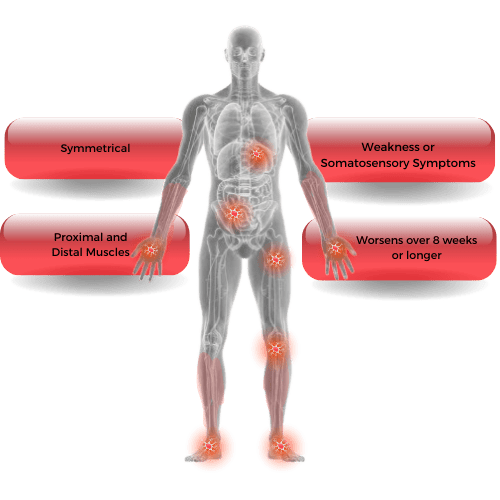
 A neurological disorder that usually affects slightly more men than women typically between the ages 40-60, and characterized by symmetrical bilateral weakness of proximal and distal muscles and impaired sensory function, including loss of reflexes.
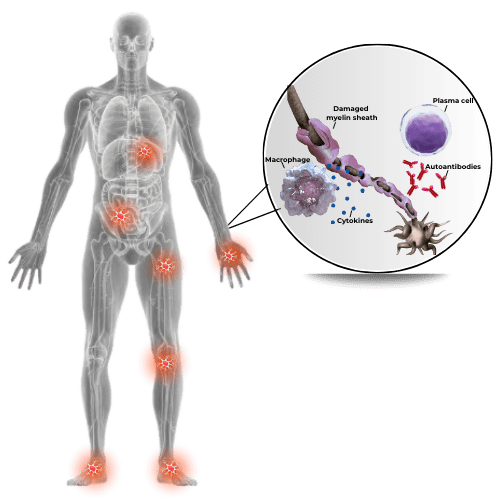
A neurological disorder that usually affects slightly more men than women typically between the ages 40-60, and characterized by symmetrical bilateral weakness of proximal and distal muscles and impaired sensory function, including loss of reflexes. - Considered an autoimmune disorder, in which the immune system attacks the peripheral nerves leading to damaged myelin and resulting in impeded signal conduction.
- The exact mechanisms by which this process happens are still not clearly defined. The antigenic target is unknown in the majority of CIDP, and no reliable biologic markers have been identified, adding to the complexities of managing this disorder.
- Incidence: Approximately 0.33 per 100,000 individuals per year.
- Prevalence: Approximately 2.81 per 100,000 individuals, however may be as high as 9 per 100,000.
- Clinical presentations of CIDP can be similar to other neuropathies, making diagnosis challenging. For example, CIDP has a similar presentation to Guillain-Barre Syndrome (GBS), except that GBS is acute and usually peaks within 4 weeks, whereas CIDP is chronic (> 8 weeks).
- The course varies widely, where some may have a monophasic course of CIDP followed by recovery, others may have progressive or relapsing-remitting courses.
- The European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) 2021 guideline now simplifies the clinical definition of CIDP diagnosis into two categories, which are typical CIDP and CIDP variants. See Guideline section for more information.

Neurology
Have a Question? Use Our New Chat Feature! Chat with our Medical Information Specialists by clicking on the Chat with us now available in the lower right corner of this screen.
Our world-leading immunoglobulin (Ig) franchise includes an intravenous and a subcutaneous option and is the cornerstone for CSL Behring's neurology therapeutic treatments. Our efforts in this area focus on bringing trusted products and technologies to serve patients with a rare and serious neurologic disease: Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
CIDP
 What are the symptoms of CIDP?
What are the symptoms of CIDP?
- progressive, symmetric weakness of the arms and legs, especially in proximal muscles; muscle atrophy
- loss of sensation (numbness); abnormal sensations (tingling, buzzing)
- progressive or relapsing loss of reflexes (areflexia)
- unsteady gait (usually resulting from sensory loss and weakness)
Supportive diagnostic tests:
- electrophysiological features of demyelination (e.g., reduced conduction velocities)
- high protein levels in spinal fluid analysis
- Inflammation, demyelination and remyelination observed in nerve biopsy samples
The symptoms of CIDP are caused by damage to the myelin sheath of peripheral nerves, but the particular nerves affected can vary from one patient to another.
Induction treatment:
- Intravenous immunoglobulin (IVIg) and corticosteroids are strongly recommended in typical CIDP and CIDP variants in the presence of disabling symptoms
- Plasma exchange is similarly effective and recommended but may be more difficult to administer and less well tolerated
- First-line therapies should be tried individually before considering combination therapy
- Immunosuppressant or immunomodulatory drug can be considered if active disease is persistent, despite the above treatment
Maintenance treatment:
- If the first-line treatment is effective, continuation should be considered
- Subcutaneous immunoglobulin (SCIg) and IVIg are strongly recommended as maintenance treatment in patients with active disease who are responsive to IVIg
- Once maximum response has been achieved, the dose/interval can be altered to find the lowest effective maintenance dose
Supportive therapy:
- Neuropathic pain should be treated according to published guidelines
- Advice about foot care, lifestyle management, and patient support groups should be considered
Note:
A patient’s response to treatment should be monitored carefully and continuously. The primary goals of treatment for CIDP are to:
- reduce symptoms (weakness, sensory loss, imbalance, pain)
- improve functional status (reduce disability and handicap)
- maintain long-term remission, if possible
Without treatment, approximately 30% of CIDP patients will progress to wheelchair dependence. Early recognition and proper treatment can prevent loss of nerve function and avoid a significant amount of disability.
![]() INCAT
INCAT
Background
The Inflammatory Neuropathy Cause And Treatment (INCAT) disability score was developed in 2001 and used for the first time in a clinical study comparing the efficacy and safety of intravenous immunoglobulin with oral prednisone in patients with CIDP. It has subsequently been used in several CIDP clinical trials, although its use in day-to-day clinical practice is uncommon.
Scoring
The INCAT comprises two parts, the arm score and the leg score. Based on a patient’s level of impairment in their arms and legs, each part is scored between 0 and 5 points, resulting in an INCAT total score between 0 and 10. The adjusted INCAT score, which is used in clinical trials, excludes changes of 0 to 1 or 1 to 0 in upper limb function as regulatory agencies do not considered this clinically significant.
Note: INCAT score is inversely related to function, with 0 representing no functional impairment and 10 representing inability to make any purposeful movement with either arms or legs.
![]() I-RODS
I-RODS
Background
The Inflammatory Rasch-built Overall Disability Scale (I-RODS) was developed in 2011 and is intended to specifically assess activity and social participation limitations in patients with inflammatory neuropathies. It is a patient-reported outcome measure, meaning the assessment is dependent on a patient’s own assessment about his or her disability.
Scoring
I-RODS is a 24-item scale, with each item representing a common, daily activity. I-RODS captures clinically meaningful changes over time and is scored from 0-100 (0 is the most severe limitation in activities and social participation, 100 is no limitation in activities and social participation).
The items range in difficulty from very easy (“reading a newspaper/book” and “eating”) to very difficult (“standing for hours” and “running”) The patient assigns a score between 0 and 2 to each item as follows:
- 0 = impossible to perform
- 1 = performed with difficulty
- 2 = easily performed
![]() MRC SUM SCORE
MRC SUM SCORE
Background
The Medical Research Council (MRC) system for testing and grading of muscle function aims to provide a standardized and objective way to assess muscle function.
It was originally introduced in 1943 and has a long history of use in neurology, rehabilitation and general medicine examinations.
Scoring
In the MRC grading system, each tested muscle is assigned one of the following scores based on its function. Scores range from 0-80; 0 being paralysis and 80 being normal strength.
- 0 = paralysis
- 1 = only a trace or flicker of muscle contraction
- 2 = muscle movement is possible with gravity eliminated
- 3 = muscle movement is possible against gravity
- 4 = muscle strength is reduced, but movement against resistance is possible
- 5 = normal strength
Assessments of muscles are done bilaterally, meaning that for each muscle tested, the same muscle on the opposite side of the body is also tested. The MRC sum score is finally calculated by adding the score of each individually assessed muscle.
![]() Grip Strength
Grip Strength
Background
Grip strength assessments have been recommended to be used in clinical practice because they can be done with relatively simple tools and provide near instantaneous results.
A variety of tools are available to assess grip strength; two commonly used instruments are the Martin Vigorimeter and Jamar Dynamometer.
Scoring
With the Martin Vigorimeter, the patient squeezes a rubber ball that is connected to a manometer with rubber tubing.
- The patient’s grip strength is expressed in kilopascal (kPa), with a range of 0–160 kPa
With the Jamar Dynamometer, the patient squeezes the handle of a hand-held device inwards against increasing resistance.
- The patient’s grip strength is expressed in kg or lb, with a range of 0–90 kg or 0–200 lb
The European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) 2021 guideline defines Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) as:
- Typical CIDP
- CIDP Variants
- Distal CIDP
- Multifocal/Focal CIDP
- Motor CIDP
- Sensory CIDP
CIDP diagnosis is determined based on:
- Clinical criteria, which consists of patterns of sensory disturbance and weakness
- Electrodiagnostic criteria should be considered in addition to clinical criteria, which includes motor and sensory conduction studies
- Supportive criteria should be considered if clinical criteria is fulfilled but the electrodiagnostic criteria suggests possible CIDP. These include:
- Response to treatment (improvement on at least one disability and one impairment scale)
- Imaging (ultrasound and MRI [both are not recommended in pediatric patients])
- Cerebrospinal fluid analysis (should only be considered in cases of acute or subacute onset, and to rule out infections)
- Nerve biopsy (only recommended to diagnose CIDP under specific circumstances, i.e. determining an alternative diagnosis)
- Immunologic testing (monoclonal gammopathy testing, antibodies)
The European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) 2021 guideline provides recommendations for CIDP diagnosis and treatment and aims to individualize treatment to improve patient outcomes.. Please refer to Treatment section for more information.



