Hemophilia B
Have a Question? Use Our New Chat Feature! Chat with our Medical Information Specialists by clicking on the Chat with us now available in the lower right corner of this screen.
Educational Materials
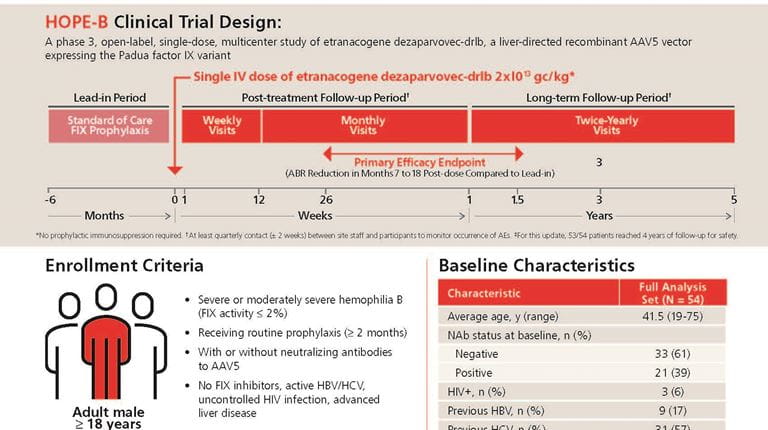
HEMGENIX HOPE-B 4-Year Data Illustration
An educational illustration highlighting the 4-year data for the HOPE-B clinical trial for etranacogene dezaparvovec-drlb. The data includes results for bleeds, factor IX activity levels, factor consumption, and safety.
Preparation and Administration
HEMGENIX should only be prepared and administered by trained staff at an administration center. Please refer to the full Prescribing Information for detailed steps on preparation and administration of HEMGENIX.
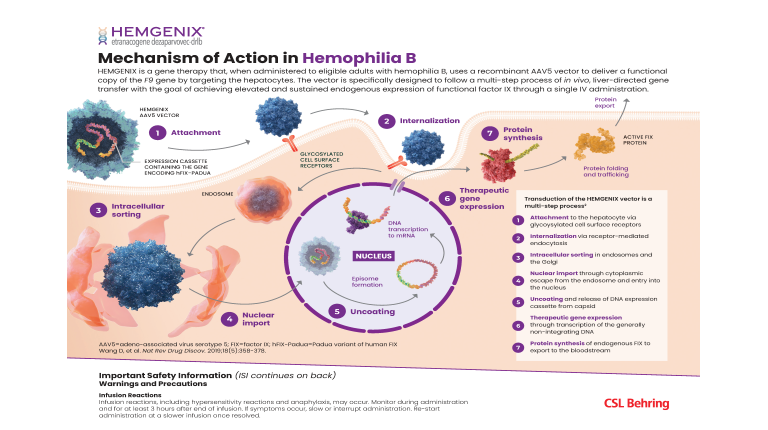
Gene Therapy Mechanism of Action
Learn about the mechanism of action (MOA) for gene therapy in hemophilia, in which liver-directed gene transfer introduces a therapeutic gene to achieve durable expression of functional factor protein to restore hemostasis.
Ancillary Flash Card
HEMGENIX Ancillary Flash Card
Important Safety Information for HEMGENIX
Warning and PrecautionsInfusion Reactions
Infusion reactions, including hypersensitivity reactions and anaphylaxis, may occur. Monitor during administration and for at least 3 hours after end of infusion. If symptoms occur, slow or interrupt administration. Re-start administration at a slower infusion once resolved.
Hepatotoxicity/Hepatocellular Carcinoma
Post-dose, monitor for elevated transaminase levels. Consider corticosteroid treatment should elevations occur. The integration of liver-targeting AAV vector DNA into the genome may carry the theoretical risk of hepatocellular carcinoma development. For patients with preexisting risk factors for hepatocellular carcinogenicity, perform regular (eg, annual) abdominal ultrasound and alpha-fetoprotein testing following administration.
Immune-mediated neutralization of the AAV5 vector capsid
Preexisting neutralizing anti-AAV antibodies may impede transgene expression at desired levels.
Monitoring Laboratory Tests
In addition to monitoring liver function, monitor for Factor IX activity and Factor IX inhibitors after administration.
Adverse ReactionsThe most common adverse reactions (incidence ≥5%) were elevated ALT, headache, blood creatine kinase elevations, flu-like symptoms, infusion-related reactions, fatigue, nausea, malaise, and elevated AST.
Indication for HEMGENIX
HEMGENIX®, etranacogene dezaparvovec-drlb, is an adeno-associated virus vector-based gene therapy indicated for the treatment of adults with Hemophilia B (congenital Factor IX deficiency) who:
- Currently use Factor IX prophylaxis therapy, or
- Have current or historical life-threatening hemorrhage, or
- Have repeated, serious spontaneous bleeding episodes.
HEMGENIX is for single use intravenous infusion only.
Contraindications: None.Please see full prescribing information for HEMGENIX.
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.